
A simplified definition would run as follows: in food chemistry, any heating steps involving the presence of sugars and amino compounds (ammonia, amino acids, proteins or polypeptides) lead to the complex reactions originally demonstrated by Louis-Camille Maillard (1878-1936) ca. 1912. Maillard demonstrated to scientists at the French Academy the formation of a yellowish-brown colored liquid, via mixing amino acids and sugars. That the discovery failed to knock the academy scientists off their chairs is now well known and, likely in part due to two world wars, his work (1912-16) was not resurrected until the 1950’s.
Since then the Maillard reaction has gained worldwide interest from both the food and medical fields with relevance to food flavor and coloring, digestibility of food and due to the toxicity of certain Maillard compounds and associated implications for several diseases. The modern era of research, starting in 1953, is attributed to a J. E Hodge who first defined three stages or phases to the Maillard process and then unraveled some of the overall complexities of the scheme within each stage.
When the Maillard, non-enzymatic “browning reactions” take place they lead to the formation of reducing substances and a plethora of flavorful compounds which include; malty, toasted, bready and nutty flavors. The reducing substances, which were termed reductones, were shown to exhibit antioxidant behavior and thus to reduce the number of damaging oxidation reactions which occur during product aging. Brewers and maltsters have been aware of Maillard-type reactions since the early 1900’s even prior to Maillard’s own demonstrations. With respect to the malt kilning process the following provides a neat and familiar description:
“The products obtained from the former are sugars and from the latter amino-compounds. When these amino-compounds are heated at 120—140° with sugars such as ordinary glucose or maltose, which are produced at this stage of the process, combination occurs. The precise nature of the compounds produced is unknown to me, but they are probably glucosamine-like bodies. When heated at the temperature to which the malt is subjected at the final curing stage these compounds are decomposed, forming dark coloured substances” Ling (1908).
Clearly the Maillard reaction (as will be understood from the discussion that follows) is at play here in the quote. Moreover, it is known that darker beers made with the more highly roasted malts have more reducing potential, due to higher reductone content (also noted below), and thus longer shelf-life stability.
Modern day brewers now have access to an ever-wider variety of grains, cereals and other ingredients that may have been “touched” by the magic of Maillard chemistry and they and maltsters should thus understand more about this topic.
THE MAILLARD REACTION
A brief discussion of the overall Maillard scheme now follows. See Figure 1 for the initial steps and Figure 2 for a general outline and key to subsequent individual steps, stages and sub-reactions.
Figure 1. Many sugars and many amino acids lead to 1000’s of Maillard compounds.
As noted above, the Maillard reactions are initiated by the condensation (the joining together with the loss of water) of a carbonyl group of a reducing sugar and an amino compound. Depending upon the raw material source there are many sugars available to react and nineteen common amino acids plus one imino acid (proline) present which may lead to 100’s if not 1000’s of chemical components in the initial phase (Stage 1 – see Figure 2) of the Maillard chemistry. For example, the reaction of glucose with the simplest amino acid glycine alone can yield 24 different compounds!
Some sugar chemistry:
Aldose – a monosaccharide (simple sugar) with an aldehyde chemical group (-CH=O)
Ketose – a monosaccharide (simple sugar) containing a ketone group (C=O)
Reducing sugar – sugars with a free aldehyde or free ketone group can act as a reducing agent and are thus called reducing sugars.
Some amino/imino acid chemistry:
Amino acid – an organic compound – building blocks of proteins and important in other biological reactions, containing amine (-NH2) and carboxyl (-COOH) functional groups and a side chain (R-group) specific to each one. If the amine group is replaced by a -C-NH group, the compound is designated as an imino acid. There are 19 common amino acids and one common imino acid – proline with a cyclic rather than linear structure. In proline, the nitrogen is “locked up” in the ring hence only one hydrogen is directly attached to the nitrogen.
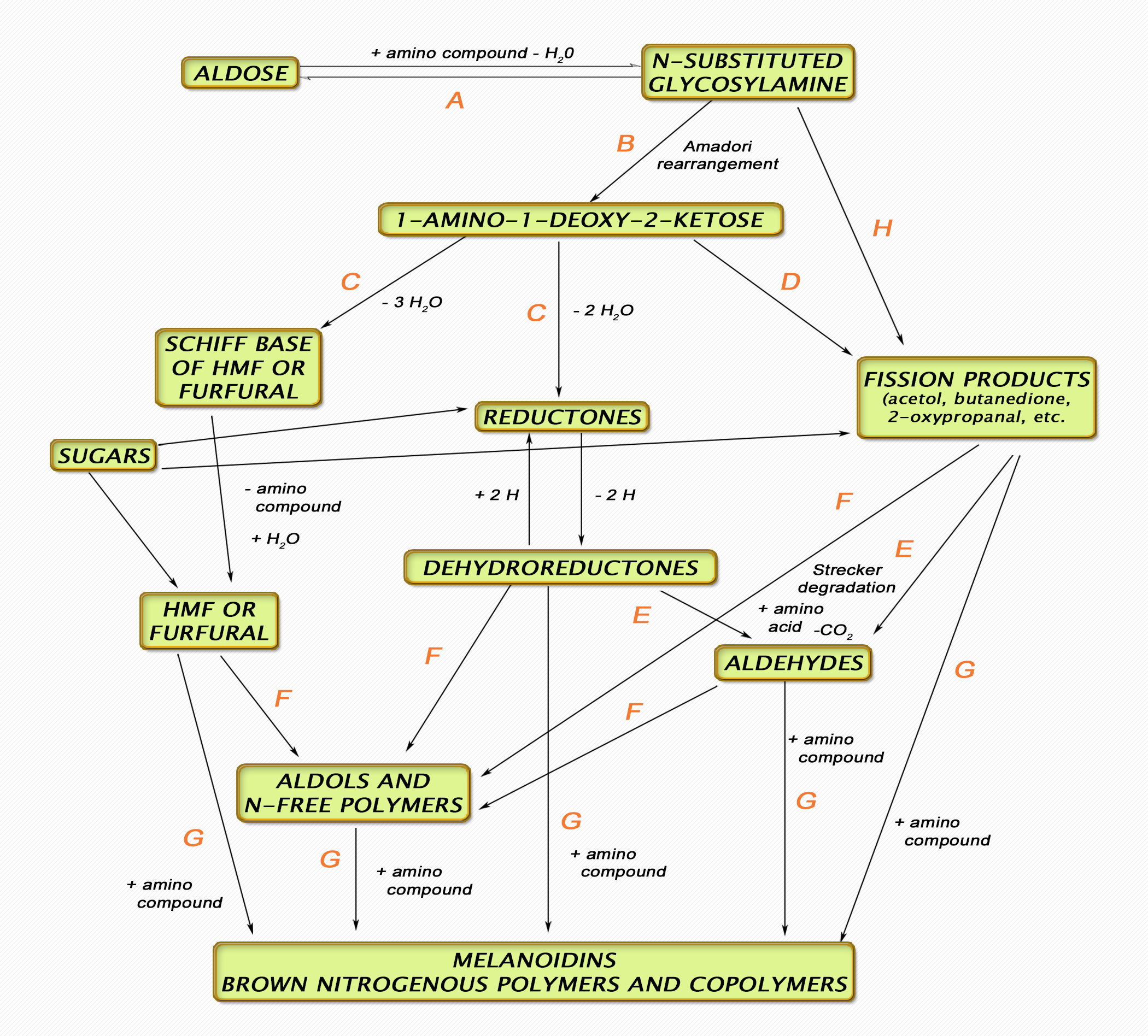
Figure 2. A simplified schematic of the processes involved in the Maillard reaction.
Three stages are considered:
Stage I: A – Sugar-amine condensation, B – Amadori rearrangement.
Stage II: C – Sugar dehydration, D – Sugar fragmentation, E – Amino acid degradation (Strecker degradation).
Stage III: F – Aldol condensation, G – Aldehyde-amine condensation and formation of heterocyclic nitrogen compounds.
Recent information adds H to the list which represents free-radical breakdown of Maillard intermediates (not covered further in this review).
These reaction steps are discussed further in the text. Adapted from Hodge (1953) as referenced in Nursten (2005).
Following the initial condensation step, a series of complex degradations, rearrangements and other reactions lead to the generation of many compounds which influence both the color and flavor of foods and beverages. The additional reactions lead to many important classes of flavor compounds including aldehydes and heterocyclic molecules including furans, pyrazines, pyrroles, oxazoles, thiophenes, thiazoles and other heterocyclic compounds. These components and the process in simplified form are discussed below.
The sugar amine condensation reactions lead to relatively unstable glycosylamines (sugar-amine compounds) which then undergo a reaction known as an Amadori rearrangement. Amadori rearrangements are acid or base catalyzed isomerizations or rearrangement reactions of the N-glycoside of an aldose or the glycosylamine to the corresponding 1-amino-1-deoxy-2-ketose. Amadori reactions, which can occur spontaneously at a temperature as low as 25°C, are generally considered irreversible. Sometimes the term Heyns rearrangement will be seen in the literature and this refers to a situation whereby ketoses are rearranged to the corresponding 2-amino-2-deoxyaldoses (in place of the ketoses). These reactions and many more are described in exquisite detail by Nursten (2005).
The Amadori and the Heyns rearrangement products (ARP’s and HRP’s) are regarded as important intermediates of the early phase of the Maillard reaction. Aminoketones can react with each other to form various cyclic compounds called pyrazines (more on this later) characterized by potent flavor notes and which may be responsible for harsh and burnt flavor notes. Amadori and Heyns reaction products (ARPs and HRP’s) are, on one hand, regarded as relatively stable intermediates and have been detected in various heat-processed foods. That said, at higher pH values, ARPs and HRPs easily undergo cleavage of the carbohydrate chain, yielding fission products such as 2,3-butanedione (diacetyl), acids, aldehydes and many other components; ARP’s and HRP’s are thus good precursors for Strecker aldehyde formation. Such is the complexity of sugar chemistry including fragmentation!
Of importance to this discussion is the plethora of compounds that are produced in the intermediate stage of the Maillard scheme. See Figure 2, where sugar dehydration (Symbol C), sugar fragmentation (D) and amino acid (Strecker) degradation reactions (E) are portrayed. The amino ketoses, through the removal of 3 molecules of water, may pass through an intermediate called a Schiff base to produce the compounds furfural (caramel, sweet and nutty nuances) and hydroxymethylfurfural (HMF) through an amino compound and further water molecule elimination. Sugars can also be converted to furfural and HMF via other routes. Note: these two compounds are also thus produced during caramelization.
Sugars and, through sugar dehydration reactions, amino ketones can form reducing compounds known as reductones, which themselves can reversibly interconvert to dehydroreductones via hydrogen addition or elimination. Reductones are products formed from the loss of two molecules of water, unlike when three waters are removed which leads to the furfurals (as noted above). Also in the intermediate stage (or stage 2) the well-known Strecker degradation reactions occur. Strecker degradation is primarily a major pathway for the conversion of amino acids into structurally related aldehydes of significant flavor value. The amino acids are degraded by dicarbonyl compounds, also formed in the Maillard reaction, leading to deamination (amino group removal) and decarboxylation (CO2 elimination) of the amino acid. Aldehydes (and this also applies to ketones) are produced with one less carbon atom than they started with. The amino group is transferred to the other reacting species in the reaction. A listing of Strecker aldehyde flavor notes can be seen in Table 1.
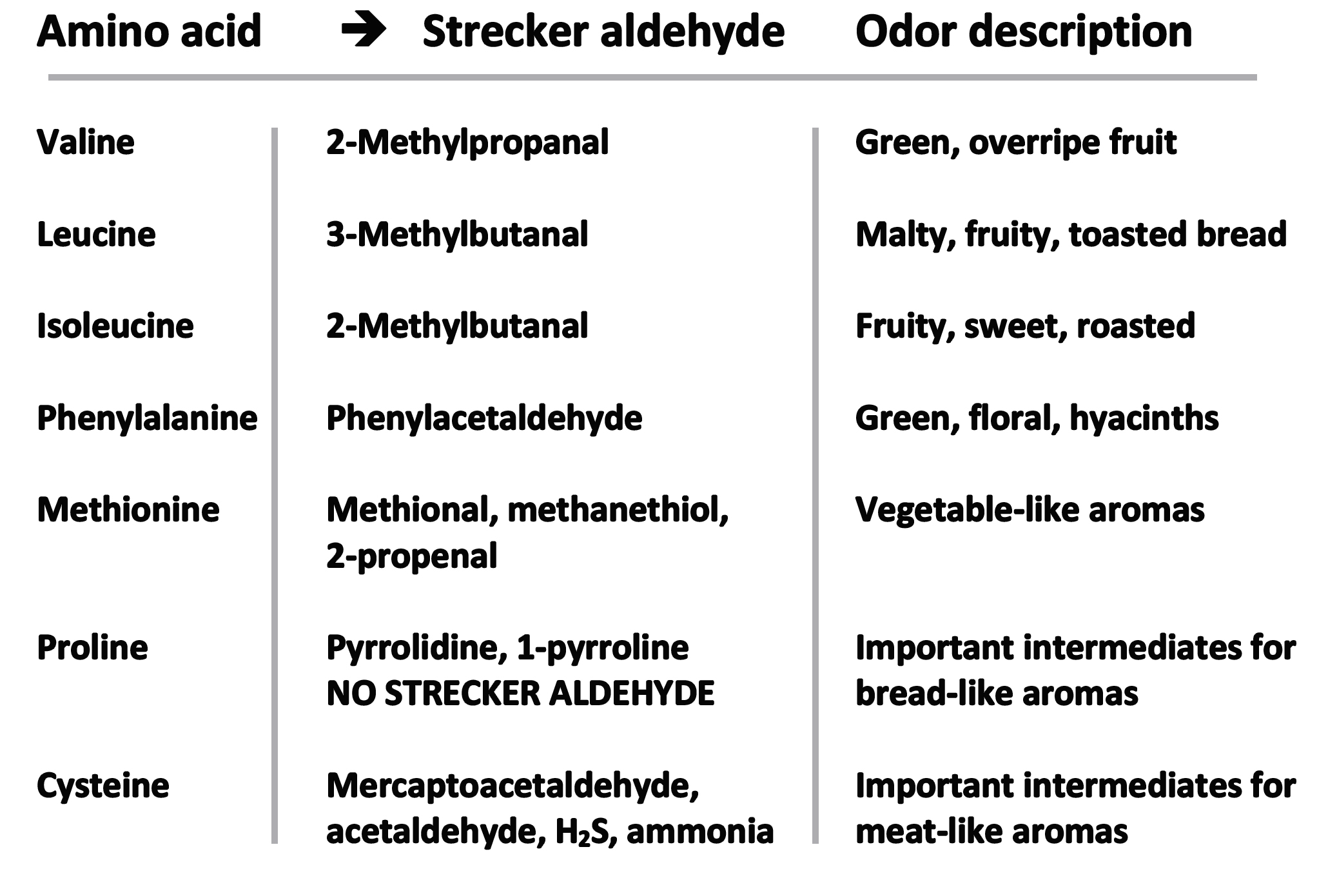
Table 1. Selected examples of amino acids, Strecker aldehydes and their associated odors.
As seen in Table 1, a veritable profile of fruity, floral, grainy, sweet, roasted, vegetable and bready and meaty flavors are associated with compounds of the chemical class known as aldehydes. Note also that the cyclic (ring-containing) imino acid proline does not degrade to a Strecker aldehyde. Proline instead forms other ring-containing molecules including the cyclic secondary amine tetrahydropyrrole (aka. pyrrolidine). The sulfur containing amino acid cysteine degrades into potent compounds including ammonia and hydrogen sulfide (rotten egg-like). Remember there are many other amino acids thus increasing the number of flavors which may be produced.
Now for coloration and flavor production we move to the more interesting final stage (stage 3) of the Maillard scheme (reactions F and G). Fission products, furfural and HMF, dehydroreductones and aldehydes, through aldol condensation reactions (F), result in aldols and nitrogen-free polymers; these reactions represent a very large and complex area of chemistry (again see Nursten {2005} for more detail). An aldol is an abbreviation for an aldehyde and an alcohol – an organic compound containing an alcohol and a carbonyl group, especially a compound known as a beta-hydroxy aldehyde. Moreover, by aldol condensation of two sugar fragments or a sugar fragment and an amino acid fragment, heterocyclic aroma compounds are generated upon cyclization, dehydration and/or oxidation reactions. See Figure 3 and associated discussion for more on heterocyclic chemistry.
Aldehyde-amine condensation reactions and formation of heterocyclic nitrogen compounds are illustrated in the reactions marked G in Figure 2 (see also Figure 3). The very final products of non-enzymatic browning are complex high-molecular weight brown nitrogen-containing polymers and copolymers known as melanoidins to distinguish them from the melanins produced by enzymatic browning. Unfortunately, the dark brown melanoidins have proven difficult to isolate, characterize and identify based on their overall complexity, though are considered responsible for the uniquely characteristic rich toasty-sweet notes in bocks, doppelbocks and other heavy-malt accented beers.
From the above discussion, it is seen that a cascade of reactions produce a vast pool of compounds. These arise from early product rearrangements and then degradation, elimination, cyclization, dehydration, fission and fragmentation reactions. These many reactions all lead to a multitude of flavor and color compounds.
Another major class of Maillard-associated chemical components, generated through cyclization reactions, is seen in Figure 3. This figure shows heterocyclic compounds which play a key role in beer and distilled spirits flavor. Simply defined, heterocyclic compounds are ring-like structures which contain atoms other than carbon in the ring; oxygen, nitrogen and sulfurs primarily being present in such molecules.
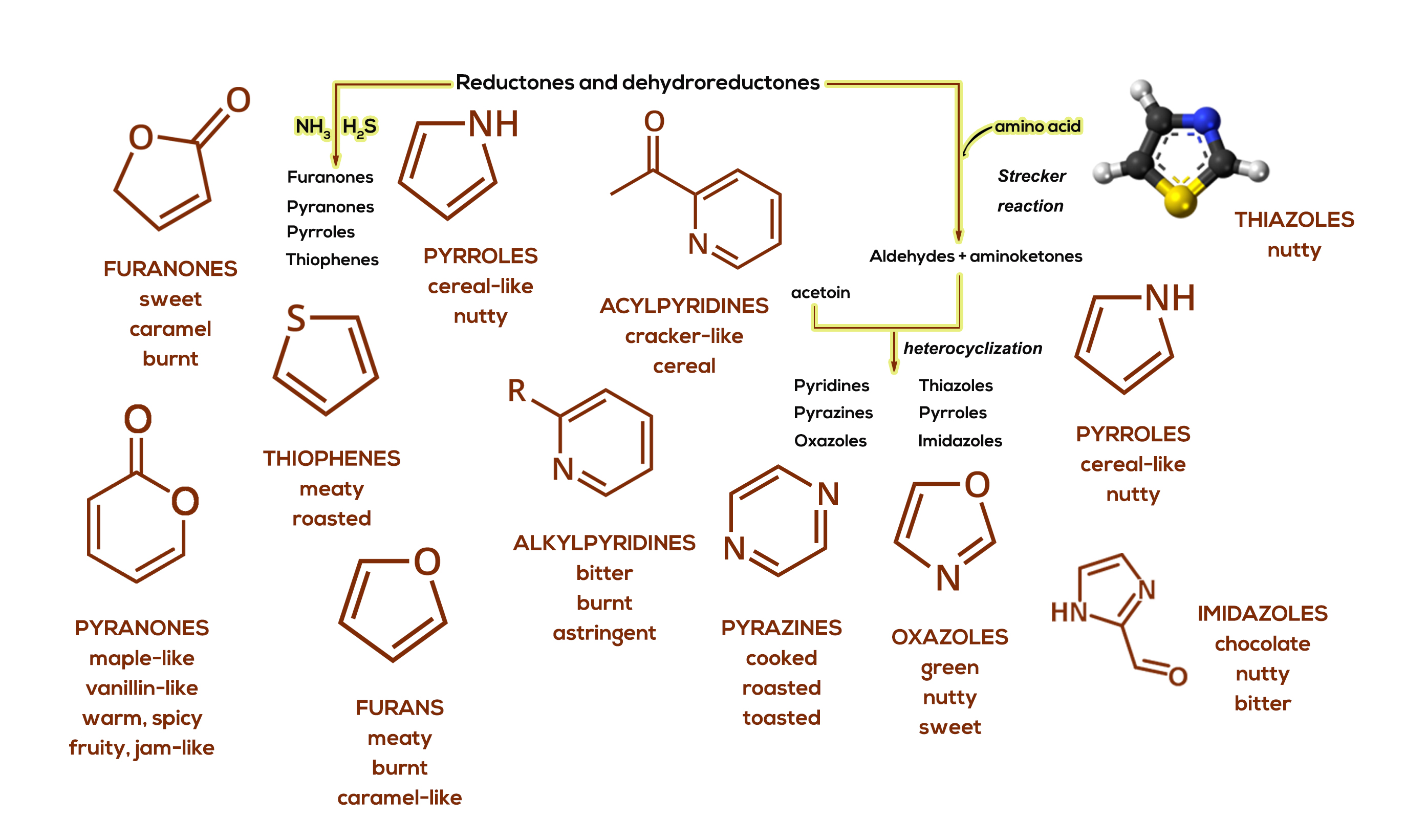
Figure 3. A simplified schematic of the processes involved in the formation of heterocyclic compounds via Maillard chemistry.
Through reductone and dehydroreductone chemistry, and their resultant product interactions with ammonia NH3 and hydrogen sulfide H2S, the heterocyclic compounds; furanones (oxygen in the ring), pyranones (oxygen), pyrroles (nitrogen) and thiophenes (sulfur) are produced. The basic structures and general flavor notes associated with these compounds can also be seen in Figure 3. More significantly, each of these base heterocycles can have many different chemical substituents attached, thus vastly increasing the total number of possible compounds and potential flavor nuances detected in foods and beverages.
Figure 3 also shows a few more details of the Strecker degradation reaction scheme. Interactions of aldehydes and aminoketones with a compound called acetoin can lead to formation of pyrazines (dual nitrogen atom ring heterocycles), pyridines (nitrogen), oxazoles (oxygen), imidazoles (dual nitrogen’s in the ring) and thiazoles (sulfur). Once again, we note that base (simple skeleton forms) and more complex substituted heterocycles are formed. Furans (five membered oxygen-containing ring heterocycles) are also illustrated in Figure 3. They arise from rearranged sugars. Once again, note the main flavor characteristics for each type of heterocycle from this part of the scheme. These components may be present in very small amounts yet, with very low detection thresholds, can quite significantly impact beverage flavor profiles.
CONDITIONS FOR THE MAILLARD REACTION.
As the Maillard process is a series of chemical transformations, factors that influence a chemical reaction also affect the Maillard reaction. The rates of chemical reactions depend primarily on temperature, pressure, time and concentration of reactants. Maillard reaction products increase with increasing temperature, with longer heating time and at pH values above 7.0. The relative moisture content is also important and metal ions such as copper and iron have been noted as stimulating the reaction. As temperature is a key condition for the Maillard reaction further details are presented in Table 2. Some similar products to Maillard compounds are produced during the caramelization process but we note here that caramelization reactions, unlike Maillard reactions, require the input only of sugars not amino acids. The topic of caramelization could form the basis of a paper of its own and we only point out here that, at higher temperatures (see Table 2), caramelization can interfere or compete with Maillard reactions.
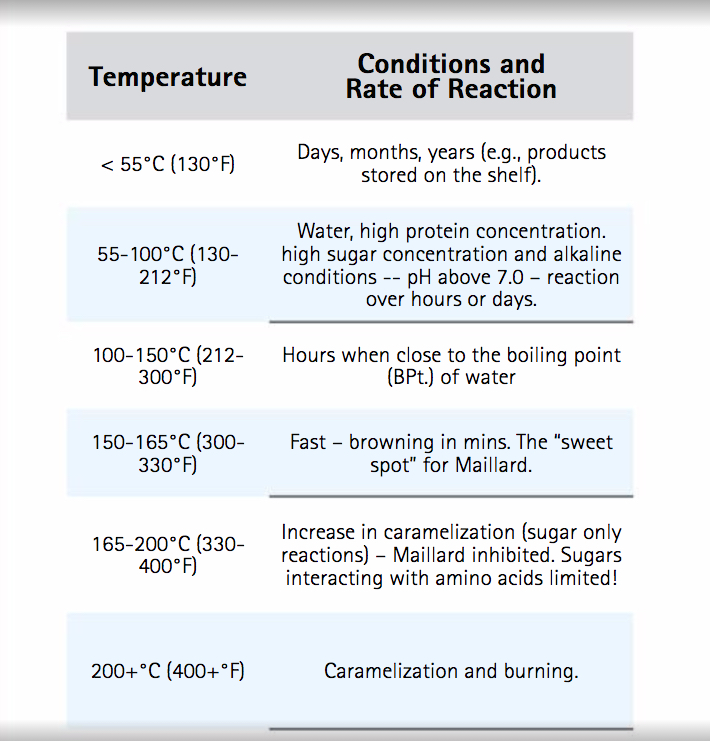
Table 2. Temperature and the rate and extent of Maillard reactions.
Finally, with respect to pH it is noted that the amount of reactive amino acids increases with pH and this thus leads to an enhanced Maillard reaction sequence at higher pH values. However, when Maillard reactions then occur the result is a lowering of the pH – the pH drops by formation of acids (through sugar degradation) and by the production of basic heterocyclic compounds. Further information regarding the conditions impacting and controlling the Maillard reaction has been presented by Ames, (1990).
BEER PRODUCTION AND THE MAILLARD REACTION.
From raw materials and from start to finish, in processing of beer, the Maillard reaction is likely to be involved at any stage involving significant heat input. Though the production and use of malt and other cereal grains is likely the most significant contributor to Maillard-derived flavor compounds. Even maltsters do not yet have a complete picture of Maillard chemistry and its full contribution to beer color and flavor. Much more research needs to be done here!
Malting/kilning: For cereal-based beers and malt beverages, the malts and other grains used provide the initial input of Maillard reaction components – the sugars (from starch degradation) and amino acids (from proteolysis). Amino acids in green malt are transformed, in a pH dependent manner, to corresponding Strecker aldehydes (see Table 1). Model Maillard reactions in the laboratory have also revealed the classic cereal, corny and bready-like flavors that originate through the complex cascade of Maillard reactions. Brewer’s malts are often produced through variable high temperature kilning and roasting which leads to many specialty grains with a wide range of flavor compounds (including caramel, roasted, nutty, burnt, coffee and chocolate). The brewer today thus has a choice of dozens of specialty malts and many other cereal grains. The full complexity of Maillard chemistry and grain production is far from being completely understood, especially in relation to the set of high molecular weight color and flavor compounds noted above – namely the melanoidins.
Cooking and mashing: Different cereal cooking systems are available to brewers, depending upon the need to gelatinize starches in non-barley malt grains and adjunct cereals. These cooking steps are of course run at high temperature. In addition to Maillard color and flavor compound formation, degradation or consumption of fermentable carbohydrates and amino acids occurs which may impact the amount of alcohol produced. Fermentable sugars are reduced in concentration – perhaps not in a huge way – but free amino nitrogen (FAN) concentration is also reduced which may also impact fermentation and thus alcohol production. Heavy adjunct sugar use will increase one of the two main reactants at the expense of nitrogen and here the FAN concentration may be too low for subsequent fermentation. Mashing operations may also produce Maillard compounds and impact ethanol yields for similar reasons noted above.
Wort Boiling and Maillard reactions: Wort contains high levels of reducing sugars and enough amino components which can continue to participate in Maillard reactions during wort boiling. Maltose and maltotriose are the most abundant sugar compounds in wort and thus, these sugars and oligosaccharides form their own specific compounds induced by Maillard reaction chemistry. One main flavor compound generated by the Maillard reaction found in wort is furfural.
This compound has frequently been regarded as an indicator for heat damage of wort. Strecker degradation reactions are also notable in the wort boil, producing the types of flavorful aldehydes as already discussed. Some of the Strecker aldehydes of low odor/flavor threshold detection concentrations are also highly volatile and may be evaporated off significantly with efficient wort boiling systems. If the brewer keeps the steam pressure during boiling at a low level, the corresponding temperature of the heating surface will be moderate and the occurrence of extensive Maillard reactions can be reduced. The use of the modern wort forced convection system is known to generate less Maillard reaction products when the steam temperature is lowered in comparison to conventional boiling. The brewer thus has a lot of potential when it comes to manipulating the extent of the Maillard reaction during the wort boiling process. More on this has been discussed by Spedding, et al, (2016).
Concluding remarks: A neat, though complex, chemistry known as the Maillard reaction has been detailed here. We hope that this review has reduced some of the mystery of this oft noted, though little discussed, reaction as applied to brewing. Once again, a key understanding of flavor origins and changes can allow ultimate control of beer quality, and a big contributor to color and flavor is to be found through understanding Maillard chemistry. It started with a yellow-brown colored product and a French chemist back in 1912. Now it is up to the brewer to see what browning and Maillard flavor production can and does do for them. Let the brewery cooking begin!
References:
Ames, J. M. (1990). Control of the Maillard reaction in food systems. Trends in Food Science & Technology. December 1990; 150-154.
Ling, A.R. (1908). J. Inst. Brewing. 14(6); 494-521.
Nursten, H. (2005). The Maillard Reaction: Chemistry, Biochemistry and Implications. The Royal Society of Chemistry.
Spedding, G., Weygandt, A. and Linske, M. (2016). Basic Quality Management – Wort Boiling. Getting Set for Efficient Fermentation – Part 2. Scandinavian Brewer’s Review. 73(4); 36-39.
